Introduction
Designated as a public health crisis by the Infectious Diseases Society of America, 10% of patients who undergo medical implant surgery will experience a bacterial infection, resulting in nearly 100,000 deaths per year in the United States (Ahmed et al., 2019). Part of the reason these infections are so difficult to treat is that the bacteria form biofilms. Biofilms consist of surface-associated assemblages of bacteria and their layered structure has shown remarkable resistance to penetration and mitigation by antibiotics (Balcázar et al., 2015; Craft et al., 2019).
Although there are many issues with administering traditional antibiotics to these surfaces, it may be possible to engineer the implant surface itself such that it will be resistant to the formation of a biofilm. Some natural surfaces, such as insect wings, lotus leaves, and shark skin, have shown a nanostructured surface that is biofilm resistant (Bandara et al., 2017; Elbourne et al., 2017; Pogodin et al., 2013). This is due to the unique nanoscale architectures that are present on the surfaces of the insect wings. These architectures are often in the shape of pillars or ridges that disrupt the ability of bacteria to attach and form communities. The structures mechanically disrupt biofilm formation, rather than using chemical means of killing the bacteria. Synthetic surfaces may be able to replicate this effect (Jenkins et al., 2020; Tripathy et al., 2017). The following experiment focused on carbon-infiltrated carbon nanotubes (CICNT), whose unique nanostructure has been previously shown to be biofilm resistant (Morco et al., 2021). These properties are currently being studied in our lab.
Carbon nanotubes (CNT) are nanostructured cylindrical molecules of hybridized carbon atoms. They are of interest in a variety of applications due to their impressive structural, mechanical, and electrical properties (He et al., 2013). CNT can be grown on titanium substrates following the deposition of thin films such as alumina and iron. A titanium substrate was chosen because titanium alloys, such as the Ti6Al4V used in this experiment, are commonly used as implant materials. The prepared substrate then goes through chemical vapor deposition process where the substrate is exposed to a carbon-rich gas at high temperatures (Speranza, 2021). Following this growth process, a post-processing carbon infiltration step can be added, depositing amorphous carbon on the nanotubes, resulting in carbon-infiltrated carbon nanotubes (CICNT). This infiltration step increases the diameter of the nanotubes, while preserving the general texture of the surface.
The CICNT surface has already shown some promise in regard to its antibacterial effects but in order to be an effective implant material it must be noncytotoxic to eukaryotic cells. When an implant is placed, bone cells known as osteoblasts deposit a layer of collagen and adhere to the surface. After time, this matrix calcifies and some of the bone cells remain behind. The matrix and the cells form new bone (Mavrogenis et al., 2009). However, in order for this process to occur, the surface must be noncytotoxic to the cells. The data found in the following experiment explores the effect that the CICNT surface has on human osteoblast cells in comparison to a bare titanium surface.
Materials and Methods
Sample Generation
The titanium alloy chosen was medical grade Ti6Al4V due to its use in orthopedic implants. Identical titanium (Ti) coupons were obtained by cutting 0.5 mm sheet stock into 9 mm x 9 mm squares, which were subsequently used for all treatment and control groups.
12 chips of Ti were sonicated for 20 minutes in isopropyl alcohol. They were then rinsed in deionized water and air dried. Six were set aside to act as controls. The remaining six Ti chips were placed in an e-beam for deposition of an initial barrier layer of 180 nm of alumina, followed by deposition of 6 nm of iron catalyst layer using a thermal evaporator. After deposition of the alumina and iron layers, the chips were placed into a furnace at 800 ˚C for a nanotube growth phase using ethylene and hydrogen gas for 1 minute. An infiltration step followed at 900 ˚C for 8 minutes in ethylene and hydrogen. The CICNT-coated chips were cooled for use in the cell quantification experiment.
hFOB 1.19 Cell Culture
An orthopedic implant would be expected to come into contact with osteoblasts, which form bone. A cell line of human osteoblasts (hFOB 1.19, ATCC CRL-11372) was chosen to investigate the response of the CICNT surface to osteoblastic cells. Cells were grown at the permissive temperature of 34°C and were cultured in a 1:1 mixture of Ham’s F12 Medium and Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum, 0.3 gm/mL of G418, and 1% penicillin and streptomycin. Cells were passaged every 2-3 days.
CellTiter Glo ATP Assay
A luminescent ATP Assay (CellTiter-Glo 2.0, Promega) was used to quantify ATP to determine the number of surviving osteoblastic cells on the experimental samples. All cells generate ATP such that the amount of ATP directly correlates to the number of living cells. The assay lyses the eukaryotic cells and generates a luminescent signal, which is proportional to the amount of ATP present.
Six CICNT and six bare Ti chips were placed in a 24-well plate and sterilized using 70% ethanol, followed by three washes in phosphor-buffered saline. Cells were seeded onto the chips at a concentration of 105 cells/mL. A serial dilution to form a standard curve was also performed in the same well plate. 24 hours later, the ATP assay was added to the plate according to the manufacturer’s protocol and the solution was transferred to a 96-well opaque plate to be read by a Victor Nivo plate reader (PerkinElmer).
Statistics
Results were evaluated using Student’s t test.
Results
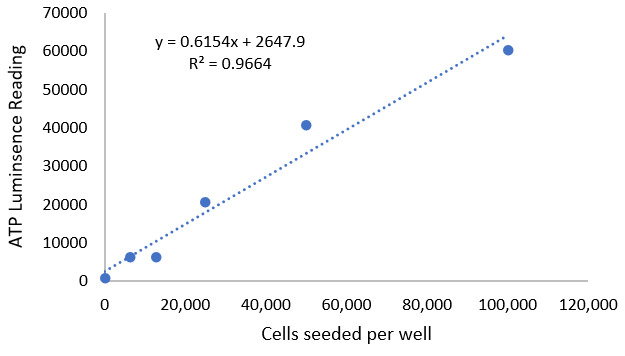
A standard curve control (Fig 1) was performed with the osteoblast cell line for this experiment. The standard curve was determined by performing a serial dilution of the cells and using the ATP assay to quantify them, showing that the luminescence reading is directly proportional to the number of cells present. This proportional relationship was used to determine the number of viable cells on each surface given the luminescence reading from the ATP assay.
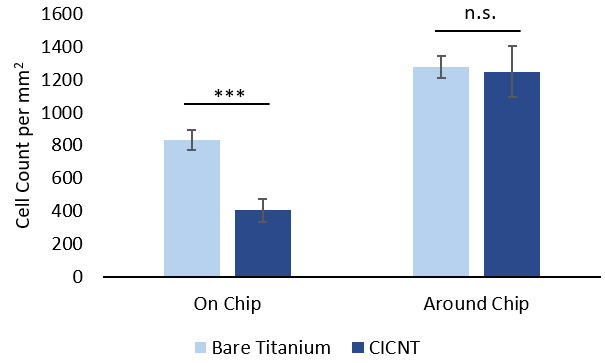
Human osteoblasts were cultured on both CICNT chips and bare titanium chips and quantified using the ATP assay, as described above. Luminescence readings were correlated to cell counts using the relationship generated by the standard curve. As expected, it was observed that the plastic well around the chips supported more cells than either bare titanium or the CICNT surface (Fig 2). The number of cells on the bare titanium chips was found to be larger than the number of cells growing on the CICNT chips (Fig 2).
Discussion
The goal of this experiment was to determine whether the CICNT surface is habitable for human osteoblasts, considering that carbon nanotubes have known antimicrobial properties which may also be toxic for human cells. The results indicate that there was approximately a two-fold reduction in number of viable cells on the CICNT surface as compared to a bare titanium surface. The titanium alloy used in this experiment, Ti6Al4V, is a commonly used material for medical implants due to its high biocompatibility and mechanical strength. Despite the reduction in number of cells on CICNT compared to bare titanium, the experiment showed that coated CICNT surfaces allow osteoblast survival over the time period studied. It can be concluded that CICNT metal is potentially biocompatible, indicating that CICNT holds promise as a viable surface for medical implants.
Carbon-infiltrated carbon nanotubes have been shown to possess antimicrobial properties (He et al., 2013). Antimicrobial surfaces are important because the infection burden in medical implants is high and projected to grow [13]. Knowing that osteoblasts potentially adhere to CICNT surfaces, future research can be focused on comparing bacterial adherence to bare titanium and the CICNT surface, as well as assessing eukaryotic cell function. The CICNT surface is a promising modification for current implant materials due to its proposed properties of eukaryotic cell survival, biofilm resistance, and mechanical strength from the substrate.
Limitations of the current study include the short timeframe of the experiment (24 hours). Longer amounts of time could allow for more cellular attachment and growth, or the opposite could occur. Future work will investigate the response of human osteoblasts to the CICNT surface over a longer period of time. In addition, this experiment assessed only the number of viable cells present on each material, and future work will also assess cellular function and metabolism.
Conclusion
Carbon-infiltrated carbon nanotubes offer a potential surface modification for medical implants. Current materials, such as titanium, allow the growth of bacterial biofilms, which are difficult to treat and cause significant issues for patients. An ideal implant surface would physically prevent the formation of bacterial biofilms while remaining a habitable surface for human bone cells. This experiment showed that viable osteoblasts remained on the CICNT surface after 24 hours. Future work will continue to explore cellular function and metabolism on this unique surface.