Introduction
Carbon nanotubes (CNT) consist of cylindrical sheets of graphite “grown” in nanoscale diameters. They have sparked a multitude of uses in materials engineering since they were discovered in 1991 (Iijima, 2002). There are many different applications of CNT, including in electrochemical biosensors (Wang, 2005), drug delivery (Ilbasmiş-Tamer et al., 2010; Ménard-Moyon et al., 2010) pharmaceutical production (Rode et al., 2018), cancer research (Sonmez et al., 2016), tissue engineering (Alshehri et al., 2016; Gerasimenko et al., 2019), and treatments for the brain and nervous system (Parpura, 2008). CNT are particularly valuable for their electrical, chemical, and mechanical properties (Guignier & Bueno, 2019).
CNT are created using a chemical vapor deposition (CVD) (Endo et al., 2006) process where a gas containing carbon elements, such as ethylene (C2H4), is reacted with a substrate material with catalytic particles such as iron, to form carbon nanotubes (Zhang et al., 2013). This reaction occurs at very high temperatures and is made possible using a specialized CVD furnace.
CNT can undergo a post-processing carbon infiltration step, which deposits bulk carbon around the nanotubes, greatly increasing their diameter, and resulting in carbon-infiltrated carbon nanotubes (CICNT). CICNT is a desirable surface modification because it has previously been shown that CICNT exhibit structural antibiofilm activity (Morco et al., 2021). This would be extremely useful in the medical field since bacterial biofilms cause serious issues for patients and are extremely difficult to treat with traditional antibiotics. The antibacterial effects of the CICNT are presumed to be due to its unique nanostructure, which may mechanically disrupt the attachment of bacteria. Nanostructures on natural materials such as insect wings have been previously discovered to be antibacterial (Jaggessar et al., 2017), and CICNT may act in a similar manner.
Ti6Al4V Substrate
The biocompatible titanium alloy chosen for this research, Ti6Al4V, has been commonly used as a biomaterial in modern medical applications. It is an attractive implant material due to its advanced corrosion resistance along with desirable physical properties including a low elastic modulus, high strength, and durability. These qualities, in addition to the potential antibacterial properties of CICNT, makes Ti6Al4V a material of interest for medical implants.
Methods
Identical samples of Ti6Al4V titanium were obtained by cutting 0.5 mm sheet stock into 1 cm width squares. The samples were then cleaned by sonication for 15 minutes in isopropyl alcohol, whereupon they were rinsed with deionized water and air dried. After sonication, three samples were set apart as control samples, and twelve samples were coated with an alumina layer. The alumina barrier layer was deposited in one of two ways: atomic layer deposition (ALD) and physical vapor deposition (PVD). For both deposition methods, two different alumina layer thicknesses were used: 50 nm and 30 nm, with three samples in each treatment group.
Atomic layer deposition
The first form of alumina deposition tested was atomic layer deposition (ALD). In this method, precursor reactants were introduced sequentially to the surface in question. Over time, a thin film of alumina was deposited due to exposure to these reactants. Since the reactants are introduced sequentially, ALD is extremely precise, offering extremely fine control of layer thickness, even over an uneven surface (George, 2010). 50 nm of alumina were deposited using ALD on 3 samples of Ti6Al4V in an ALD150LX (Lesker) machine. 30 nm of alumina were deposited on another three samples in the same manner.
Physical vapor deposition
The other method of alumina deposition studied was a form of physical vapor deposition (PVD) using electron beam (e-beam) evaporation. In this method, an electron beam was used to evaporate alumina under high vacuum. The evaporated alumina then condensed onto the substrate in a thin layer (Rossnagel, 2003). 50 nm of alumina were deposited using PVD on three samples of Ti6Al4V in an e-beam (Denton) machine. 30 nm of alumina were deposited on another three samples in the same manner.
Carbon-infiltrated carbon nanotube growth
After the deposition of the alumina layer, all samples went through identical subsequent treatments. A 6 nm iron catalyst layer was deposited using a thermal evaporator on all samples including the control samples. The samples were then placed into a furnace that was heated to 800 °C with hydrogen (H2) and ethylene (C2H4) gas for a one minute growth phase, flowing at 338 and 331 standard cubic centimeters per minute respectively. The purpose of the growth phase is to form the carbon nanotubes and then to increase their height. This growth phase was followed by an infiltration stage at 900 °C with hydrogen and ethylene flowing at the same rates as above for eight minutes. The infiltration of carbon nanotubes allows for the massive increase of carbon nanotube diameter by the deposition of amorphous carbon onto the previously grown nanotubes.
Following CICNT growth, each sample was imaged in a scanning electron microscope (SEM) and evaluated for nanotube coverage and nanotube diameters.
Results
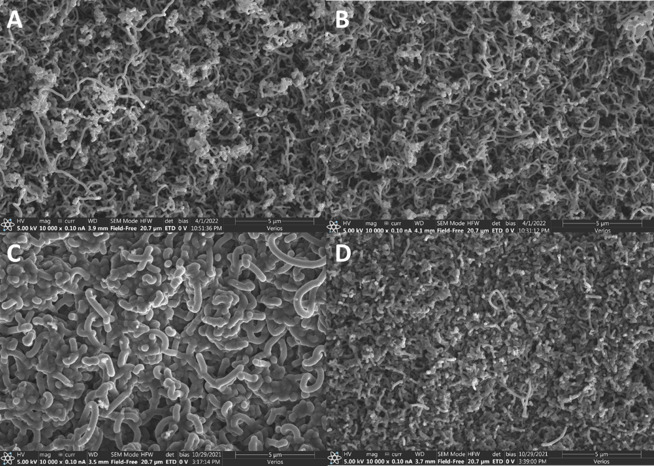
Growing CICNT on Ti6Al4V (the control samples) resulted in inconsistent, patchy growths that did not cover the titanium alloy surface (Fig 1). It was hypothesized that the addition of a barrier layer of alumina would provide a more amenable surface to nanotube growth. Thin films of a barrier layer of alumina and a catalyst layer of iron were added to the surface, and the addition of these layers resulted in consistent nanotube coverage of the substrate (Fig 1).
Additionally, it was hypothesized that the deposition method and alumina layer thickness would influence nanotube coverage. Alumina was deposited on six samples using an ALD method in two different thicknesses, 30 nm and 50 nm. Alumina was also deposited on six additional samples using a PVD method in thicknesses of 30 and 50 nm. After CICNT growth, the samples were imaged and compared for nanotube coverage (Fig 2).
Discussion
The results of these experiments showed that the deposited alumina barrier layer, in addition to the iron catalyst layer, allowed carbon nanotubes to grow on Ti6Al4V. However, no substantial difference was noted between samples with alumina from either ALD or PVD sources at any of the thicknesses studied. The nanotube coverage was even, and the diameters of the tubes were similar, except for the sample with 30 nm deposited by ALD, which had thicker nanotubes than the other samples. One explanation for this could be its location in the furnace relative to other samples, but more research will need to be done to further explore this phenomenon.
However, it is hypothesized that ALD would be more effective for 3-dimensional or irregularly shaped samples since a full coverage is atomically deposited layer by layer. Future research will consider this possibility.
Limitations of the current study include the fact that only two alumina layer thicknesses were investigated. It is hypothesized that there exists a lower limit for the barrier layer thickness, as well as potentially an upper limit due to differences in the thermal conductivity in titanium, alumina, and CICNT. It is possible that as the lower limit is neared, the method of alumina deposition will affect such a limit due to differences in the film quality of each method. Future work will continue to explore the potential existence of such limits to the thickness of the alumina layer.
It was found that a barrier layer of alumina on Ti6Al4V is required to start carbon nanotube growth. The catalyst iron layer and titanium alloy alone will not promote growth reactions for developing the carbon nanotubes, as the addition of the alumina layer is vital for CICNT growth.
Conclusion
Carbon-infiltrated carbon nanotubes are a desirable surface due to their unique nanostructures as well as their favorable antibacterial, mechanical, electrical, and thermal properties. This experiment demonstrated that CICNT can be grown on a Ti6Al4V substrate with the addition of a barrier layer of alumina. Regardless of the deposition method, the alumina layer was shown to be effective at thicknesses as small as 30 nm, as measured by nanotube coverage of the substrate. The combination of CICNT and a Ti6Al4V substrate has the potential to be important in many industries, including the development of improved medical devices.
_showing_cicnt_growth_vs_a_control_sample_wit.png)

_showing_cicnt_growth_vs_a_control_sample_wit.png)